How to Make New TB Technology More Accessible to the Private Health Sector
Editor’s note: Dr. Madhukar Pai is a Canada Research Chair in Epidemiology & Global Health at McGill University, Montreal; director of McGill Global Health Programs; and associate director of the McGill International Tuberculosis Centre. Here he answers questions about TB testing in privatized health markets.
 Dr. Madhukar Pai (left): Xpert MTB/RIF (Cepheid Inc., California) is a rapid, automated, molecular diagnostic test for TB. It is substantially more accurate than sputum smear microscopy, the conventional test for TB in developing countries. Within 90 minutes, Xpert allows for accurate detection of TB and also detects resistance to rifampicin, a key TB antibiotic. Because the system is automated and simple to use, it can be done outside of sophisticated laboratories. Xpert was endorsed by WHO in 2010, and since then, over 15 million cartridges have been procured at concessional prices in high TB burden countries.
Dr. Madhukar Pai (left): Xpert MTB/RIF (Cepheid Inc., California) is a rapid, automated, molecular diagnostic test for TB. It is substantially more accurate than sputum smear microscopy, the conventional test for TB in developing countries. Within 90 minutes, Xpert allows for accurate detection of TB and also detects resistance to rifampicin, a key TB antibiotic. Because the system is automated and simple to use, it can be done outside of sophisticated laboratories. Xpert was endorsed by WHO in 2010, and since then, over 15 million cartridges have been procured at concessional prices in high TB burden countries.
It is a big advance because Xpert has “democratized” molecular diagnostics for the developing world. Molecular tests are not new, but they were rarely used in high TB burden countries because of high costs and the need for sophisticated labs and highly trained lab scientists. Xpert is the first technology to break these traditional barriers. Also, by combining detection and drug-susceptibility testing in a single test, it has shown us that we are dealing with a lot more multidrug-resistant TB (MDR-TB) than we imagined. Thus, Xpert has opened the path for universal drug-susceptibility testing, a key component of the End TB Strategy by WHO and partners. The success of Xpert has also attracted several companies to enter the TB market.
KP: What is concessional pricing and what role does it play in TB treatment?
MP: Because the development of Xpert was supported by several donors, and because of a price buy-down that happened in 2012, the price of each Xpert cartridge is fixed at $9.98. But this price only applies to the public sector in eligible high-burden countries.
Even in poor countries with a high tuberculosis burden, the private sector is not eligible for concessional pricing for Xpert, nor for other WHO-endorsed tests such as line probe assays (LPA) and liquid cultures. Manufacturers set higher prices for reagents and instruments for private laboratories and institutions than for the public sector, and there are additional costs (such as import duties) and margins imposed by distributors, intermediaries and laboratories.
KP: Why are private sector pricing and access so important in TB care?
MP: The private sector is a major source of health care in 12 of the 22 countries with the highest tuberculosis burden, including India, Pakistan, the Philippines, Bangladesh, Afghanistan, Kenya, Uganda, Vietnam, Indonesia, Myanmar, Nigeria and Cambodia. In these economies, even poor patients with tuberculosis seek care from private health care providers, and delayed diagnosis and misdiagnosis are important problems. Further, patients do not respect public versus private boundaries and often switch between these sectors. Even doctors practise in both sectors. So, if we want to diagnose TB early and accurately, we need to think about patient pathways to care and understand that patients often begin their pathway in the private and informal sectors. Regardless of where patients seek care, we need to ensure that they have access to high quality, patient-centric TB diagnosis and treatment.
Related posts: From Pilots to Systems (Part 1) and (Part 2)
Editor’s note: For the sake of clarity and context, in the paragraphs below Pai discusses the study cited in The Lancet Global Health and its findings about the affordability and accessibility of Xpert in the private sector in high-burden countries; exceptions to those findings; and how companies and product developers might approach the TB market with a different mindset.
In our study, we contacted tuberculosis experts in 12 countries with large private sectors and asked them to check commercial availability of Xpert in their country, and collect price data from private laboratories that offer Xpert testing. We had at least two respondents from each of the 12 countries. As shown in the table below, in six of the 12 countries, there is no commercial availability of Xpert in the private sector. In the remaining six countries, the average price charged by private laboratories was U.S. $68.73 (range $30.26–$155.44).
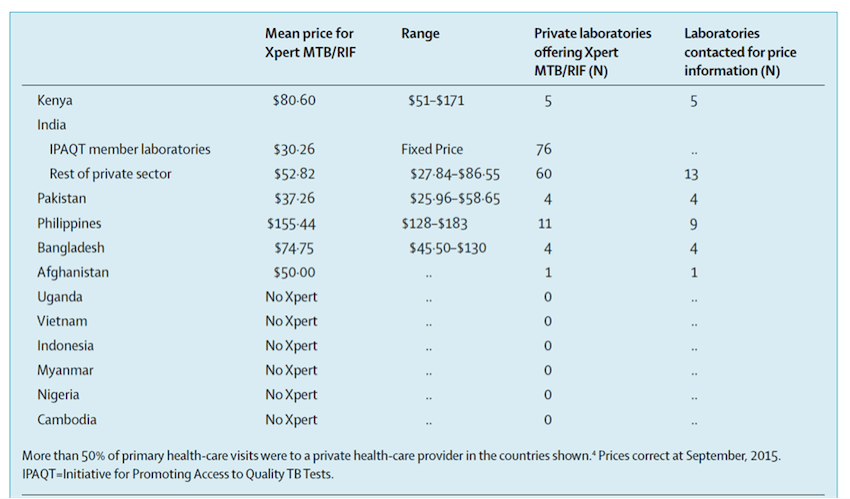
So, our survey suggests that commercial sale of Xpert seems to be limited and, with some exceptions, patients in the private sector pay a lot for this test. These factors could result in low levels of access to quality tests and blunt the benefits of new diagnostic tools.
In the countries we surveyed, the highest price for patients paying for Xpert testing was in the Philippines, while the lowest average price was offered in India, via laboratories in a network called the Initiative for Promoting Affordable and Quality TB Tests (I-PAQT). I-PAQT, a private sector initiative coordinated by the Clinton Health Access Initiative in New Delhi, offers WHO-approved diagnostics at concessional prices. Laboratories in I-PAQT offer Xpert at a fixed price of $30.26, compared with an average of $52.82 in the rest of the private sector in India. By January 2016, I-PAQT had consolidated 113 accredited private laboratories that receive concessional pricing for Xpert, LPA and liquid cultures. In exchange for access to the public sector concessional pricing for reagents and equipment, member laboratories must agree to pass on price reductions to patients, by charging no more than a transparently agreed ceiling price, notifying tuberculosis cases to the public sector, and participating in quality assurance programmes. Since its launch in 2013, more than 250,000 TB tests have been done by I-PAQT laboratories.
TB is a disease of the poor. While anyone can get TB, the disease primarily affects the poor and the vulnerable. So … product manufacturers cannot view the TB market the way they would approach diabetes or cancer. They will need to realise that most patients with TB in countries with a high burden of the disease have limited means, and a mass-market (low margin but high volume) rather than premium (high margin but low volume) pricing model may be more appropriate for TB tests in these countries. The I-PAQT experience in India supports this theory. While TB patients cannot pay a lot, the fact that over 9.5 million people develop TB every year does suggest the need to think of base-of-the-pyramid strategies to serve these large numbers of patients.
KP: What strategies might lead to more consistent access and pricing among private health care providers worldwide?
MP: Our data strongly underscore the need for a private sector access strategy to ensure that quality diagnostics reach all patients with suspected tuberculosis. (For a panel discussion on this topic, click here.) The strategy will need to draw on various approaches, such as the inclusion of the private sector in current and future pricing agreements, replication of I-PAQT-like models in other economies, consolidation of private laboratories by intermediary agencies, public-private mix projects to allow privately managed patients to be tested in public facilities, use of subsidies and vouchers by private provider interface agencies, and social businesses to cross-subsidise tuberculosis tests against more profitable tests.
KP: Are there any other TB diagnosis/treatment advancements on the horizon? What role might they play?
 MP: As described in the UNITAID TB diagnostics technology and market landscape reports, there are now several companies developing novel molecular tests for TB. One product, the next-generation version of GeneXpert, called GeneXpert Omni (left), is scheduled to be launched later this year. This is a small, portable, battery-operated, automated molecular device that can be used at the point-of-care. Cepheid, in partnership with FIND (Geneva) and partners, has also announced the impending release of Xpert MTB/RIF Ultra, a cartridge that will have higher sensitivity than the current one. My hope is that such point-of-care technologies will have the potential to reach lower-level laboratories and primary care centers, where patients with a cough often seek initial care. Unless we achieve early and accurate diagnosis, we are unlikely to interrupt TB transmission.
MP: As described in the UNITAID TB diagnostics technology and market landscape reports, there are now several companies developing novel molecular tests for TB. One product, the next-generation version of GeneXpert, called GeneXpert Omni (left), is scheduled to be launched later this year. This is a small, portable, battery-operated, automated molecular device that can be used at the point-of-care. Cepheid, in partnership with FIND (Geneva) and partners, has also announced the impending release of Xpert MTB/RIF Ultra, a cartridge that will have higher sensitivity than the current one. My hope is that such point-of-care technologies will have the potential to reach lower-level laboratories and primary care centers, where patients with a cough often seek initial care. Unless we achieve early and accurate diagnosis, we are unlikely to interrupt TB transmission.
(To see an interview Pai conducted at the William Davidson Institute – the parent organization of NextBillion – in 2013, click here.)
Lead photo courtesy of FIND: A technician in the Ssese Islands, an archipelago in the northwestern part of Lake Victoria, Uganda, using the Xpert MTB/RIF test to diagnose tuberculosis.
Kyle Poplin is the editor of NextBillion Health Care.
- Categories
- Health Care
