Launching Products that Meet Needs: Addressing market demand through user-centered development
Editor’s note: This article is part of the Market Dynamics initiative on NextBillion Health Care. The ongoing series is designed to encourage discussion and understanding around how markets impact health outcomes. This blog is the third of three that PATH is contributing to the series. Click here for the first article, wiritten by Amie Batson, about strengthening markets for reproductive health; and here for the second, written by Jane Hutchings, Seema Kapoor and Christopher Brady, about access and equity in reproductive health.
Creating and launching products that can address the health needs of women, men and children in low-resource settings is complex and challenging, particularly since markets for health products in low-income countries can be uncertain. Products generated through a holistic process that starts with good product development, and incorporates public health and commercialization perspectives throughout the process, are more likely to meet the needs of the intended user groups and other key stakeholders.
That is why at PATH we’ve adapted best practices from user-centered product development to guide our processes – from defining the problem through needs identification assessments, to establishing the product objectives and requirements, to developing and testing prototypes, and field testing and refining these until we arrive at the features and functionality needed. Throughout this process we keep the end user and their context in mind, and also factor in the needs/demands of other critical stakeholders who influence product availability and use. This requires understanding the perspectives of multiple stakeholders and using technical, public health and commercialization inputs to prioritize needs and address trade-offs to move a product forward.
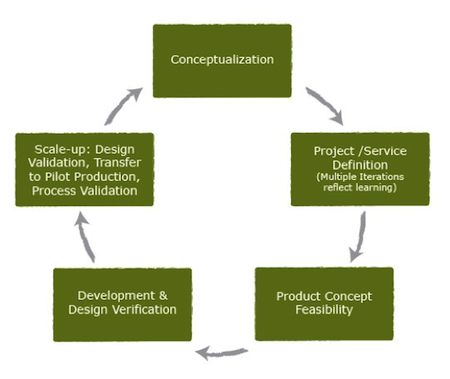 Throughout the product development process, PATH takes an iterative approach -– with continued design refinement – and works with public- and private-sector partners who bring additional skills needed to move these lifesaving technologies toward validation and market-readiness.
Throughout the product development process, PATH takes an iterative approach -– with continued design refinement – and works with public- and private-sector partners who bring additional skills needed to move these lifesaving technologies toward validation and market-readiness.
(The product development process, left. Illustration courtesy of PATH.)
PATH licenses our technologies to industry partners who bring the expertise in manufacturing, marketing and distribution required for commercialization to ensure access in developing countries. PATH continues to help pave the way for launch in low-resource settings and ensures that attention is paid to the needs of users and other stakeholders during introduction and scale-up. We work to ensure the product is delivered in a manner that is safe, appropriate and sustainable.
Expanding options for non-hormonal barrier contraception
Research exploring what women want in their contraceptive methods was the genesis for two of PATH’s products: the SILCS diaphragm and the Woman’s Condom. Women said they wanted access to more methods they could control, would have few side effects, could be easily started/stopped and could protect from sexually transmitted infections (STIs). The Guttmacher Institute’s recent analysis of unmet need for family planning —– why women who do not want to become pregnant are not using a contraceptive – reveals these issues first identified two decades ago still exist today.
SILCS diaphragm – user input reinvents an old method
PATH surveyed women’s groups, health care providers and donor agencies to better understand the market for diaphragms. We learned that women wanted a more comfortable device that is easy to insert and remove. Providers and donors wanted a product that is easier to supply, stock and provide. These performance objectives set the benchmark for SILCS designs evaluated by users.
Along the way, PATH and partners developed and tested approximately 200 prototype designs spanning six device generations. We refined the single-size diaphrag, and collected user input in four countries over 10 years to ensure that SILCS would be easy to use and comfortable. Clinical studies validated the safety, efficacy and acceptability of the single-size SILCS diaphragm.
PATH licensed the SILCS technology to a private-sector partner, Kessel medintim GmbH of Germany, for commercialization, and now this product is approved and being marketed in more than 20 countries as the Caya® contoured diaphragm. Product launch in the U.S. is expected in 2015. PATH and multiple partners are working to introduce SILCS in developing countries where diaphragms have not been available in decades while assessing the added value of combining it with microbicides – drugs designed to protect from HIV – which would allow the SILCS diaphragm to potentially protect from pregnancy and HIV.
Woman’s Condom – and good sensation
High rates of unintended pregnancy, maternal mortality and HIV infection worldwide reinforce the need for prevention options designed with women’s realities in mind. Currently, female condoms are the only woman-initiated product that offers dual protection from unintended pregnancy and STIs, such as HIV. Yet, issues with availability, uptake and price of existing female condom products persist.
PATH and our research partners and user-groups on four continents tackled the challenge of developing a female condom that is easy to use, broadly acceptable across diverse regions and provides good sensation for both partners to strengthen uptake of this product category. The Woman’s Condom now is approved in multiples countries and marketed under various brands: O’Lavie and V Condom in China and South Africa; and soon to be launched as the SafePlan Condom and Whisper female condom in Malawi and Zambia.
During product development, PATH established test sites in Thailand, Mexico, South Africa and the U.S. to gather feedback from users with diverse physical and cultural needs. Women and their partners evaluated prototypes through an iterative process, and researchers interviewed the couples on key features. We used this feedback to refine features and functionality for ease of use, comfort and performance. In total, we developed and tested more than 50 designs reflecting various solutions to user-related concern. Women and their partners helped develop a refined female condom that is easy to insert and provides good sensation for women and men. Clinical studies in multiple countries have validated the safety, acceptability and performance, and in comparative studies, women and men have preferred the Woman’s Condom over other female condoms.
PATH licensed the Woman’s Condom to the Dahua Medical Apparatus Corp. in China for manufacturing and marketing. Dahua designed and engineered specialized production equipment that simplified manufacturing and allows for expanded production. PATH continues to work with Dahua and other partners to develop markets for the Woman’s Condom in developing countries to strengthen and expand the female condom market overall, and to expand protection options for women.
Using stakeholder perceptions guide access to HIV self-testing
The availability of drugs to treat HIV infection and prevent HIV transmission has bolstered the fight against the disease. Successful treatment or prevention relies on an individual’s knowledge of his/her infection status, but as many as 65 percent of people living with HIV in sub-Saharan Africa don’t know they have the disease.
A rapid “self-test” – designed to be used in private – may present a promising solution that could address issues of stigma, lack of access and resources for facility-based testing, and other barriers that exist for HIV testing. However, self-tests for HIV are not widely available in most low-resource countries.
PATH recently examined the use of HIV self-tests in Kenya, Malawi and South Africa, and interviewed stakeholders to assess barriers and opportunities for HIV self-testing, how to accelerate access to self-testing and identify characteristics of groups most likely to use self-testing.
We also evaluated the ease of use and acceptability of different rapid test prototypes and discovered that inexperienced users resulted in high error rates. These findings indicate that design improvements to current tests to reduce errors that could contribute to incorrect results could make them appropriate for use in unsupervised settings.
Based on this research, PATH aims to guide development of the next generation of HIV self-tests. Some priorities for our development work include simpler sample-collection methods, clear labeling and pictures, and intuitive instructions that can be understood by the average user without demonstration. We believe that fewer steps and easy-to-interpret results will also help to increase the usability of HIV self-tests.
The difference between success or failure of a product or health intervention often is rooted in our ability to understand the perspectives of potential users and other stakeholders who will be affected. That’s why at PATH, we place user perspectives at the center of our product development and take time to understand the complex systems where our health interventions will be used. We look to both public- and private-sector partners to garner the right skills and expertise for development, commercialization and scale-up.
Patricia Coffey is a program adviser and leader of the Health Technologies for Women and Children group within the PATH Technology Solutions Global Program. Maggie Kilbourne-Brook is a program officer with PATH’s Technology Solutions Global Program.
- Categories
- Health Care, Technology
