‘We Can’t Be the Only Ones’ (Part 2): Jaundice-fighting D-Rev gives its views on markets and success
In Part 1 of our look at D-Rev, we talked about the San Francisco-based nonprofit’s decision to upgrade its Brilliance phototherapy device, used to treat infant jaundice in the developing world, and its manufacturing and sales partnership with Phoenix Medical Systems of India. Here, in Part 2, we delve into how D-Rev deals with barriers to scaling.
Doctors in low-income hospitals in India are used to broken equipment.
“They’ve gotten accustomed to products that don’t work,” said AJ Viola, neonatal jaundice initiative product manager at D-Rev. “When a device shows up and after two months it just stops working, they roll it into a corner. They don’t call anyone, they just say, ‘Yep, it stopped working,’ and they make do with whatever else they have.”
The problem, Viola explains, is they’re either buying cheap products or using donated products, where there’s no support system in place to make sure the equipment works as intended.
Viola says D-Rev’s “trying to change that attitude.”
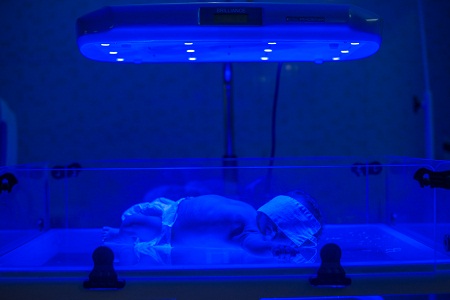 That’s a primary reason why the organization partners with India-based Phoenix, which provides on-the-ground support for D-Rev’s phototherapy models – the Brilliance Pro. which was launched this week, and its two-year-old predecessor, Brilliance Classic. Phoenix “won’t allow a device to languish in a ward,” Viola said.
That’s a primary reason why the organization partners with India-based Phoenix, which provides on-the-ground support for D-Rev’s phototherapy models – the Brilliance Pro. which was launched this week, and its two-year-old predecessor, Brilliance Classic. Phoenix “won’t allow a device to languish in a ward,” Viola said.
(Photo of the Brilliance Classic in use, left, by Tina Loveridge.)
Quality and service matter, said D-Rev CEO Krista Donaldson, but it’s also important to ensure a device is “being used the way it was designed to be used and addressing the issue it was designed to address.” While D-Rev has budgeted for 20 to 30 trips to India from San Francisco this year, and Viola himself plans to visit three or four times, Donaldson says D-Rev leans heavily on Phoenix for real-time feedback on the products’ survivability and use.
But D-Rev gives just as much consideration to “where” as “how.”
Donaldson says D-Rev thought long and hard about the specific locations where it does business in India and throughout the world. “We look not just at need but barriers to entry,” she said. That includes factors such as: How present is the aid industry and how does that skew the market? Are products being donated throughout the market? What are the registration costs? Regulatory requirements?
“We don’t think in terms of shaping markets; we respond to what a market wants,” she said. “When we think of markets, we think in terms of where are the barriers to scaling in those markets and how can we address those?”
 Viola adds: “Is there transparency in information, so the people understand the decisions they’re making in a market? And, then, are there options available?
Viola adds: “Is there transparency in information, so the people understand the decisions they’re making in a market? And, then, are there options available?
(Brilliance Classic, pictured on the left, and Brilliance Pro.)
“We’re seeing more on the options side, for sure, in India. But we still struggle with information. While a lot of folks have spent time trying to sell devices to these markets, there has not been a lot of effort to educate and train on cutting-edge research. Even something as simple as LED technology, which is what Brilliance uses and what phototherapy units in the U.S. and the West have been using for many, many years, is still a relatively new product in India. A lot of people are really stuck on the old. A lot of doctors prefer the old style, and the old technology, because that’s what they know and they’re hesitant about trying something new until there’s been more education.”
The goal, Donaldson said, is “to bring value to our users,” and that’s “an integrated process.”
“Younger entrepreneurs often do things chronologically,” she said, starting with product development and proceeding to manufacturing. “They should think about scaling and servicing as early in the design process as possible. … You can develop a product, you can make a product, you can service a product, but if nobody uses it, you don’t make an impact. Social enterprises should be thinking about that in their own design process.”
That also makes it easier to find a partner like Phoenix, she said, because it gives you a set of criteria your partner must have.
Viola says that when D-Rev is considering success and impact, it asks: “Are other folks following suit? While we think it’d be great if every hospital in India had a Brilliance unit, we would be just as happy if every hospital in India had a high-quality phototherapy device that worked. We recognize that we can’t be the only ones, that we’re not going to get 100 percent market share. But if we can motivate or entice other players to come into the market – they see us doing well, they see us picking up sales, they see us picking up market share, and they decide that, wow, there’s a market here that these guys are starting to exploit and we want to get in there – and the prices come down and the quality improves, we’d call that a success.”
There are now two generations of Brilliance lights. Will there be a third? Viola, pointing out that users are constantly demanding more and D-Rev’s always looking to reduce transportation and manufacturing costs, says “we’re always open” to that possibility.
Kyle Poplin is editor of NextBillion Health Care.
- Categories
- Health Care
